WHEN DOES ALZHEIMER DISEASE BEGIN?
We do not know how Alzheimer’s disease begins but damage to the brain can begin 10-20 years before there are any clinical signs. During the preclinical stage of Alzheimer’s disease, people are free of symptoms, but toxic changes are taking place in the brain. Abnormal deposits of proteins form amyloid plaques and tau tangles in the brain, and once-healthy neurons begin to work less efficiently. Over time, neurons lose the ability to function and communicate with each other, and eventually they die.
IN WHAT BRAIN STRUCTURE DOES ALZHEIMER’S DISEASE BEGIN?
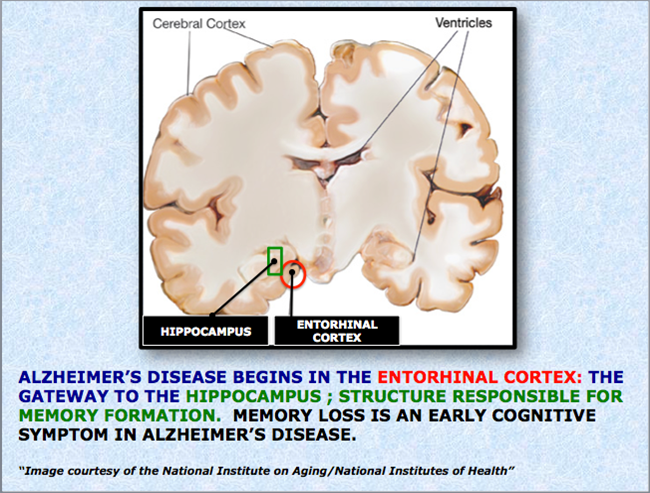
DECEMBER 22, 2013: International Headline news carried BREAKING NEWS about the exciting story that scientists from Columbia University Medical center had demonstrated where Alzheimer begins.
Scientists headed by Dr. Scott Small had once again confirmed that the Entorhinal cortex was the place in the brain where Alzheimer disease starts however this time they had accomplished this for the first time in living patients using functional MRI to map metabolic defects in the brains of living patients. After following the 96 patients for 3.5 years 12 of them developed mild AD. These 12 persons displayed significant decrease in the metabolic activity in the Lateral Entorhinal Cortex, which is the gateway to the hippocampus. This provides the first opportunity to characterize patients with AD in its earliest preclinical stages, which has significance for treatment of AD in its earliest stages.
WHAT IS GOING ON INSIDE THE ALHEIMER’S BRAIN?
There are many changes going on in the Alzheimer brain. Microscopic observations reveal changes in the Alzheimer brain after death. The three most common microscopic observations in the Alzheimer brain are: Amyloid Plaques, Neurofibrillary tangles and Loss of neuronal connections and cell death.
Read more at:
http://www.alz.org/braintour/plaques_tangles.asp
http://www.nia.nih.gov/alzheimers/publication/2011-2012-alzheimers-disease-progress-report/
MICROSCOPIC FEATURES AD BRAIN: When the harmless becomes harmful
i. AMYLOID PLAQUES
1984: Researchers George Glenner and Caine Wong identify “a novel cerebrovascular amyloid protein” known as beta-amyloid — the chief component of Alzheimer’s brain plaques and a prime suspect in triggering nerve cell damage.
A protein called amyloid precursor protein (APP) is a normal protein associated with the cell membrane.
APP is harmless when it cut up by an enzyme known as alpha secretase. This causes the release of soluble APP which is considered to be neuroprotective as well as essential for neuronal plasticity.
APP becomes harmful when it is cut up by first by an enzyme known as beta secretase and then by gamma secretase this causes the release of a fragment called beta amyloid (Aβ) peptide. It is sticky in nature and begins to stick with other fragments of beta amyloid. These sticky beta amyloid fragments begin to pile onto to each other and collect outside the neuron eventually forming an insoluble mass, which becomes the amyloid plaque.
Several types of plaques have been described:
- Diffuse plaques are focal poorly marginated collections of aggregated Aβ peptide that are not fibrillar and that lack dystrophic neurites, glial reaction, or any organized internal architecture
- Neuritic plaques contain an attenuated central core of fibrillar Aβ peptide and have neighboring dystrophic neurites They are associated with the degeneration observed at the synaptic junction and are surrounded by reactive astrocytes and activated microglial cells. Neuritic plaques also frequently are associated complement factors and immunoglobulins and a surrounding halo that includes fiber like structures.
- Burnt-out plaques represent an end stage process and feature a condensed amyloid core without associated neurites.
(Plaques description in following reference F.J. Wippold II, N. Cairns K. Vo, D.M. Holtzman and J.C. Morris Neuropathology for the Neuroradiologist: Plaques and Tangles AJNR Am J Neuroradiol 29:18–22 Jan 2008 www.ajnr.org)
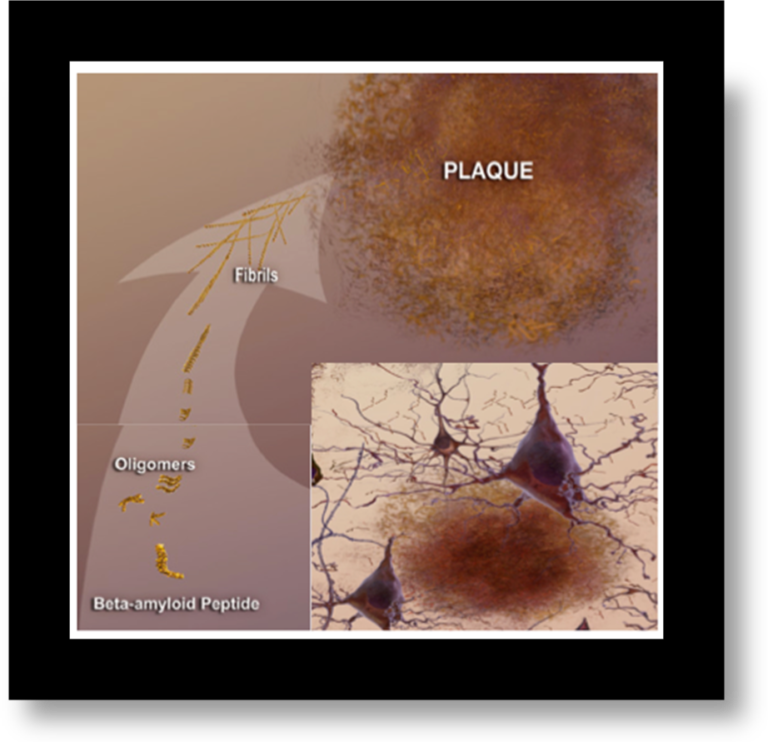
Formation of amyloid plaques. The sticky beta amyloid clumps together and eventually forms insoluble plaques outside the neuron. Neurons are being damaged by the presence of plaques as connections are lost.
“Image courtesy of the National Institute on Aging/National Institutes of Health”
ii. NEUROFIBRILLARY TANGLES
1986: Researchers discover that tau protein is a key component of tangles — the second pathological hallmark of Alzheimer’s disease and another prime suspect in nerve cell degeneration.
NEUROFIBRILLARY TANGLES (NFTs):.
TAU is a microtubule-associated protein normally located to the axon, where it physiologically facilitates the axonal transport by binding and stabilizing the microtubules. Tau in Alzheimer’s disease gets what we call hyper-phosphorylated, which means that you add phosphate groups to the protein. When you do this in an abnormal fashion, the tau moves away from the microtubule and will aggregate. You will get multiple tau proteins coming together and clumping up, inside the cell. It is believed that this clumping causes the neuron to become stressed and eventually die. Neurofibrillary tangles are found inside neurons and are twisted fibers of an abnormally processed TAU
(Professor Donna Wilcock http://www.dnalc.org/view/2173-Neurofibrillary-tangles.html)
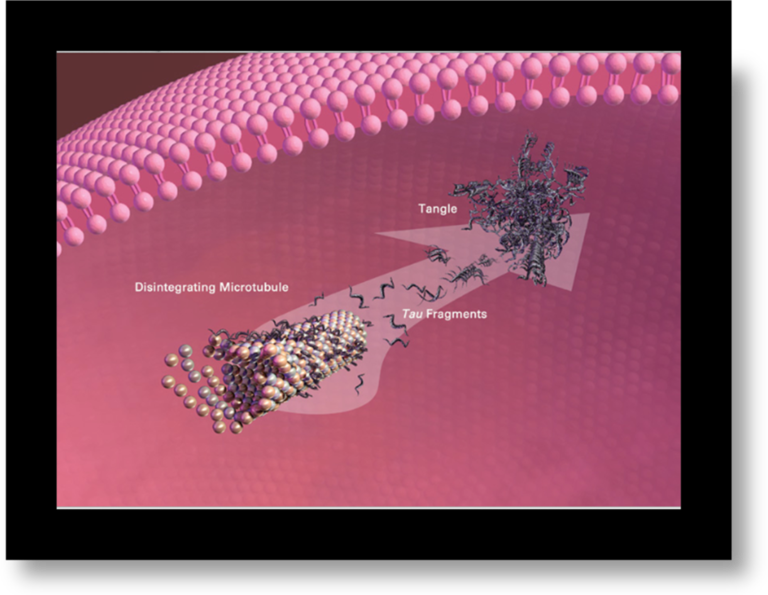
Formation of Neurofibrillary tangles which eventually causes neuronal death.
“Image courtesy of the National Institute on Aging/National Institutes of Health”
NFTs exist in three morphological stages :
- Pre NFTs which are defined by a diffuse sometime punctate tau staining within the cytoplasm of normal looking neurons.
- Mature or fibrillar intraneuronal consist of cytoplasmic filamentous aggregates of tau that displace the nucleus toward the periphery of the soma and often extend to distorted-appearing dendrites and to the proximal segment of the axon;
- Extraneuronal “ghost” NFTs (eNFTs) result from the death of the tangle-bearing neurons and are identifiable by the absence of nucleus and stainable cytoplasm
(Alberto Serrano-Pozo et al 2011)
iii. LOSS OF NEURONAL CONNECTIONS AND CELL DEATH
The third major feature of AD is a lost of connections between neurons. The progression of the plaques outside the neurons and tangles inside them cause significant damage to neurons which eventually results in their death. The figure below summarizes the main events occurring in the Alzheimer ‘s brain, which lead to neuronal death.
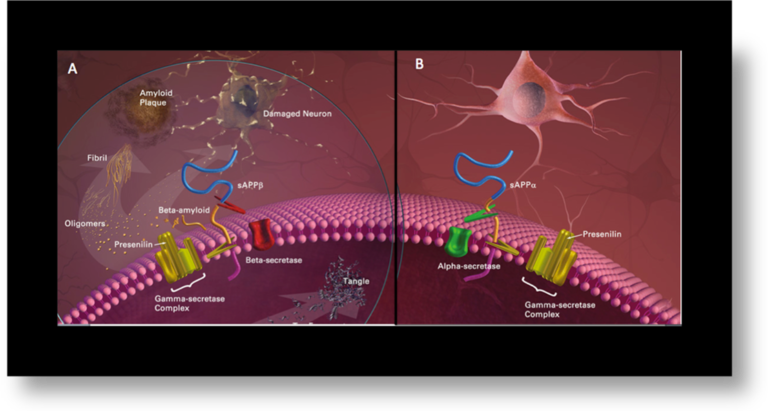
“Images courtesy of the National Institute on Aging/National Institutes of Health”
As a result of neuronal death affected brain regions shrink in a process known as atrophy. This process begins with the hippocampus, which explains why disturbances in memory occur early in the disease. Over the course of years plaques and tangles continue to spread through out the cerebral cortex (the outer layer of the brain) causing more neurons to die and further shrinkage. This progressive shrinkage in the cerebral cortex causes impairment in judgment, emotions and language.
As a result of the shrinkage persons with Alzheimer disease can lose as much as 10% of their brain mass in a 10 year period. In healthy persons over a 10 year period brain mass is reduced by 2%.
The brain begins to literally unfold. This causes the sulci to become enlarged and the gyri are decreased. The fluid filled spaces known as ventricles become enlarged.
http://www.nia.nih.gov/alzheimers/publication/part-2-what-happens-brain-ad/hallmarks-ad
http://thebrain.mcgill.ca/flash/d/d_08/d_08_cl/d_08_cl_alz/d_08_cl_alz.html
RELATIONSHIP OF PATHOLOGY TO CLINICAL PROGRESSION OF ALZHEIMER’ DISEASE
1. NEUROFIBRILLARY TANGLES
The German husband and wife team of Heiko and Eva Braak (Braak H and Braak E. 1991) graded the presence, distribution and density of NFTs tangles in the brain and defined six distinct stages of Alzheimer’s progression Braak stages. NFTs have a stereotypical spatiotemporal progression that correlates with the severity of the cognitive decline
Clinical and Pathological relations to the six Braak stages
I-II: no cognitive impairment
III-IV: no cognitive decline or mild cognitive defects
V-VI: cognitive impairment and dementia in most cases
The numbers of tangles increases as cognitive decline increases. Moreover there is a “spread” of the disease process from hippocampus where it first appears to the adjacent, phylogenetically newer neocortex in temporal lobe and then to the neocortical areas in association with cortex, such as frontal lobe.
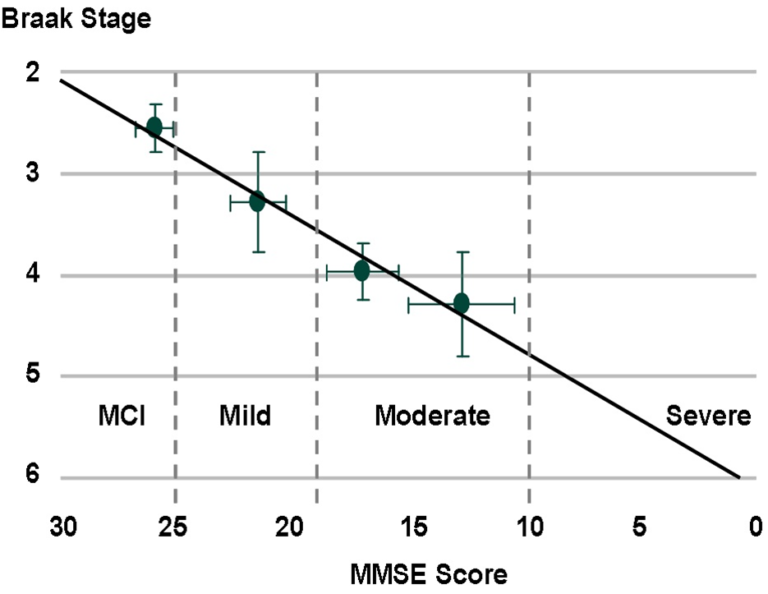
The TauRx research team built on the findings of Braak and demonstrated, in a clinical setting, that there is a strong correlation between the degree of Tau protein neurofibrillary pathology in the brain as measured using Braak staging, the amount of aggregated Tau in pre-tangle brain regions and the degree of cognitive incapacitation as measured by “mini mental state exam” (MMSE) scores http://203.116.91.52/$sitepreview/TaurxQuiz.com.sg/tau.htm
2. AMYLOID PLAQUES The neocortex is always the first region to develop Aβ deposits. Thus, it is tempting to speculate that this region has the highest susceptibility for the deposition of Aβ.
Although the spatiotemporal pattern of progression of amyloid deposition is far less predictable than that of NFTs, in general the allocortex (including entorhinal cortex and hippocampal formation), the basal ganglia, relevant nuclei of the brainstem, and the cerebellum, are involved to a lesser extent and later than the associative isocortex. The dissociation between amyloid and NFT burdens in the medial temporal lobe is particularly noticeable.
Despite the poor predictability of the progression of amyloid deposition, there is a staging of amyloid currently being used as described by Thal et al 2002 , (Alberto Serrano-Pozo et al 2011)
References
Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82: 239-259 1991
Alberto Serrano-Pozo, Matthew P. Frosch, […], and Bradley T. Hyman Neuropathological Alterations in Alzheimer Disease Cold Spring Harb Perspect Med 2011;1:a006189
Thal DR, Rüb U, Orantes M, Braak H 2002. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58: 1791–1800
